Validated Clinical Tests
Nodality develops diagnostic tests to improve clinical decisions and enhance therapeutic efficacy and efficiency. These tests use the unique capability of SCNP to reveal biology not detected by other technologies. Nodality's test development process follows design control and other best practices to meet clinical and regulatory requirements.
Nodality has clinically validated two diagnostic tests for Acute Myeloid Leukemia (AML) in samples from prospective AML clinical trials. These tests predict response to standard induction chemotherapy in patients with Acute Myeloid Leukemia (AML), one in pediatric patients and one in elderly patients (>55 years of age). The clinical trials used to develop and validate these tests were organized and performed with leading oncology cooperative groups including COG, ECOG and SWOG. Samples were prospectively collected and retrospectively analyzed in a blinded fashion.
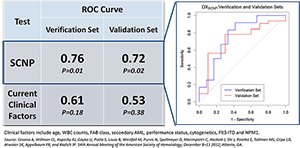
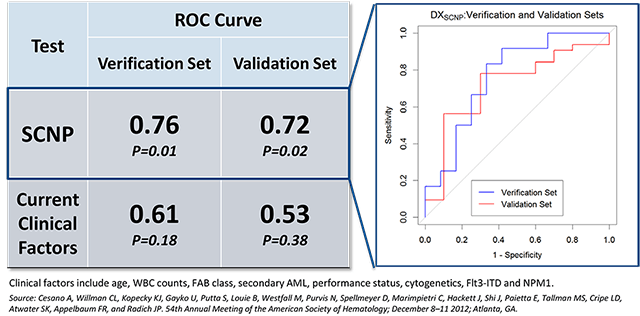
Each AML patient's blast cells have a distinct signaling network functional signature due to the signaling pathway dysregulation that leads to their disease state. For both pediatric and elderly populations, characteristic signatures were demonstrated using samples from prospective clinical trials, blinded and analyzed retrospectively, to predict which patients have a higher likelihood of responding to induction therapy and which patients have a lower likelihood of response. The latter group of patients could potentially be spared ineffective, highly toxic, and expensive treatment. In addition, those data showed that Nodality's SCNP-based test provided additional predictive value to all of the current diagnostic and patient information currently gathered by caregivers.
The second clinical application of Nodality's technology is an SCNP-based test to predict the Time to First Treatment (TTFT) in Chronic Lymphocytic Leukemia (CLL). Nodality completed multiple studies of samples from prospective trials and assayed in a retrospective, blinded fashion.
Nodality continues to develop clinical tests to improve clinical decisions and enhance therapeutic efficacy and efficiency in collaboration with its pharmaceutical, biotechnology, and diagnostic company partners.